The law of entropy is very powerful. So we cannot conserve information permanently. Any information storage will deteriorate with time and eventually be lost. However, natural selection enables genetic information to escape the law of entropy because natural selection eliminates deleterious mutations by death of living organisms.
Living Organisms Preserve Genetic Information for a Long Time
Natural selection is the driving force of evolution (1).
However, an advantageous
mutation is very rare. Usually, natural selection works to preserve genetic information (2,3,4). The
genes, which code important proteins for survival, are highly conserved throughout evolutionary time. For example,
histones, which bind to nuclear
DNA, are very important
proteins for survival. Because almost all
amino acid changes are fatal in histone H4, variants of the
peptide sequence of histone H4 are nearly always eliminated by natural selection. So the amino acid sequence of the histone H4 is almost constant during
eukaryotic evolution. The famous example, the sequence of histone H4 differs only two sites in total 102 amino acids between pea and calf. It is assumed that
animal and plant were diverged from the common ancestor about 1.2 billion years ago. This conservation of information is astounding. Continents have moved and geographic features have changed for 1.2 billion years. Any object could not remain on the surface of earth during the period. What method enables the preservation of information like natural selection? At the present day, there is no non-biological method. We shall consider why the non-biological method could not preserve information permanently and how natural selection has conserved information.
Equilibrium Is the State with Highest Probability
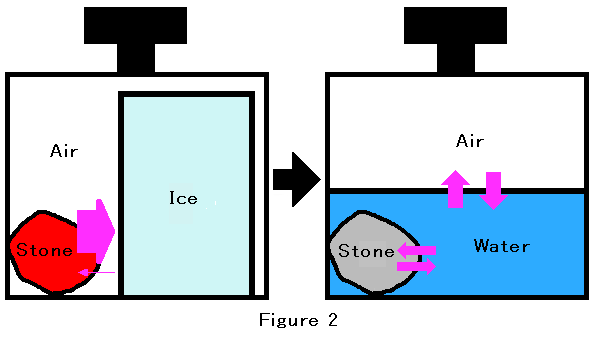
According to the second law of thermodynamics, any information will be lost for a long time. Any method of information storage needs to use deviation from equilibrium. However, the second law of thermodynamics states that any state will become equilibrium in the isolated system. Another expression is that the entropy of the isolated system approaches the maximum value. This process is based on the probability. Figure 1 shows two states. At the A state, there are two molecules of ideal gas in the left half of the room. On the other hand, there is one molecule in both halves of the room at the B state.In the initial state, the heated stone and the lump of ice are in the insulating container. During the process of approaching to thermal equilibrium, heat will transfer through the air from the heated stone to the lump of ice, and the lump of ice will be melting into water. Eventually, this closed system will achieve thermal equilibrium. After thermal equilibrium is reached, we can open the insulating container. We can pick up the stone, and then we can throw the stone into a fire. Concurrently, we can transfer water into another container, and then we can put the container into the refrigerator. After waiting for a while, we will get again the heated stone and the lump of ice. In this example, we can reverse the irreversible process. If we could use an unlimited amount of energy, we could reverse any irreversible process in the insulating container. In conclusion, an irreversible process in a closed system is not absolutely irreversible.
Natural Selection Involves the Absolutely Irreversible Process
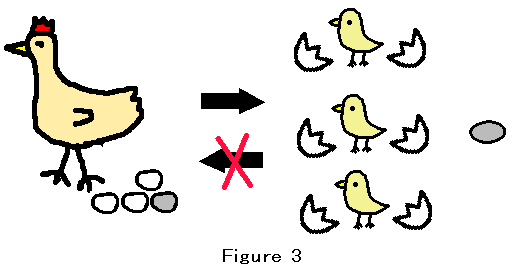
Natural selection eliminates deleterious mutations. The elimination is done by the death of living organisms. Even if we could use infinite energy, we could not revive a dead cell. The death of a living organism is absolutely irreversible. Figure 3 shows that the chicken laid four eggs and one egg had the fatal mutation.After incubation, the three eggs hatch and the mutated egg is dead. This process is irreversible. So natural selection enables genetic information to escape the domination by the second law of thermodynamics. The reason is as follows. Equilibrium is usually the balanced state. In the chemical equilibrium, the forward reaction rate equals the reverse reaction rate. Then, there is no absolutely irreversible process. If the absolutely irreversible process exists, equilibrium is not achieved.
Natural Selection Enables the Precise Copy of Information
DNA base sequence encodes genetic information. Because genetic information is very important for survival, the replication of DNA is very precise. However, if there were not natural selection, genetic information could not be preserved for a long time. Mean error rate of the DNA replication is about 1 base per 10
9 bases (5). Under the laboratory condition, Escherichia coli divides once per 30 minutes. If E. coli divided at the rate without natural selection, all bases of E. coli's DNA would change within one million years. That is, genetic information of E. Coli would be completely lost within the period. The period is very short compared with
the history of Earth. This is the power of the second law of thermodynamics. Without natural selection, the life might have ruined in a short term.
However, in the real world, natural selection has protected DNA base sequence of living organisms against the second law of thermodynamics. For example, the secondary structure of the small subunit ribosomal RNA has been extensively conserved throughout evolution (6), because it is a component of all self-replicating systems. The self-replication system is essential to life.
The oldest fossils on Earth were discovered in 3.5-billion-year-old rocks. Thus, the crucial DNA sequence of the small subunit ribosomal RNA has been conserved at least 3.5 billion years. If natural selection did not exist, this conservation is impossible.
Natural Selection Substantializes Equality among DNA Bases
DNA contains four bases:
adenine(A),
thymine(T),
guanine(G),
cytosine(C). Each base can be independently the target of natural selection. Figure 4 shows the 3'terminal three bases of E. coli's 16S ribosomal RNA gene. This is highly conserved sequence. In the figure, the ellipse represents an E. coli.
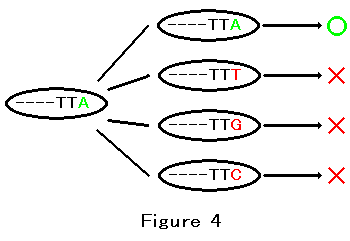
The green A represent the 3'terminal adenine. If this adenine is changed into another base, the mutated E. coli will die. The red T,G and C represent thymine, guanine and cytosine respectively. The red X represents death and the green circle represent survival. This figure represents the process of natural selection. The most important point is that an A equals to another A.
However, Socrates says in
Phaedo that any two objects are not completely equal to each other. So we have never seen two equal objects. Why do we know equality itself? Socrates answers that it is necessary for us to have received knowledge before being born. According to my interpretation, this means that we received the knowledge from our DNA, which encodes the history of evolution. That is, natural selection conserves important DNA bases: A=A, T=T, G=G, C=C. So, Richard Dawkins says that the information in DNA can be preserved forever, but only by dint of frequent re-copying (7). In conclusion, natural selection substantializes equality among DNA bases.
Natural Selection Can Protect Information against Entropy
The second law of thermodynamics or the law of entropy is very powerful. So we cannot conserve information permanently. Any information storage will deteriorate with time and eventually be lost. However, natural selection enables genetic information to escape the law of entropy. Moreover, natural selection drives evolution. If a mutation is advantageous for survival, the survival probability of the mutant organism would be larger than other organisms. Then, the mutant gene would spread in the population of the organisms, and then the mutant gene would be conserved through natural selection. That is, natural selection enables genetic information not only to be conserved but also to be accumulated, despite the law of entropy. In conclusion, natural selection can protect information against entropy.
References
1. Charles Darwin,
The Origin of Species by Means of Natural Selection 1st ed., John Murray, 1859.
2. Motoo Kimura, Evolutionary rate at the molecular level, Nature 217:624-626, 1968.
3. Motoo Kimura, The neutral theory of molecular evolution, Scientific American 241(11): 98-126, 1979.
4. Motoo Kimura, The Neutral Theory of Molecular Evolution, Cambridge University Press, 1983.
5. B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts, and P. Walter,
Molecular Biology of the Cell 4th ed., Garland Sciences, 2004.
6. C. Zwieb, C. Glotz and R. Brimacombe, Secondary structure comparisons between small subunit ribosomal RNA molecules from six different species, Nucleic Acids Research 9(15): 3621-3640, 1981.
7. Richard Dawkins, The Ancestor's Tale: A Pilgrimage to the Dawn of Evolution, Houghton Mifflin, 2004.